ISSN 0253-2778
CN 34-1054/N
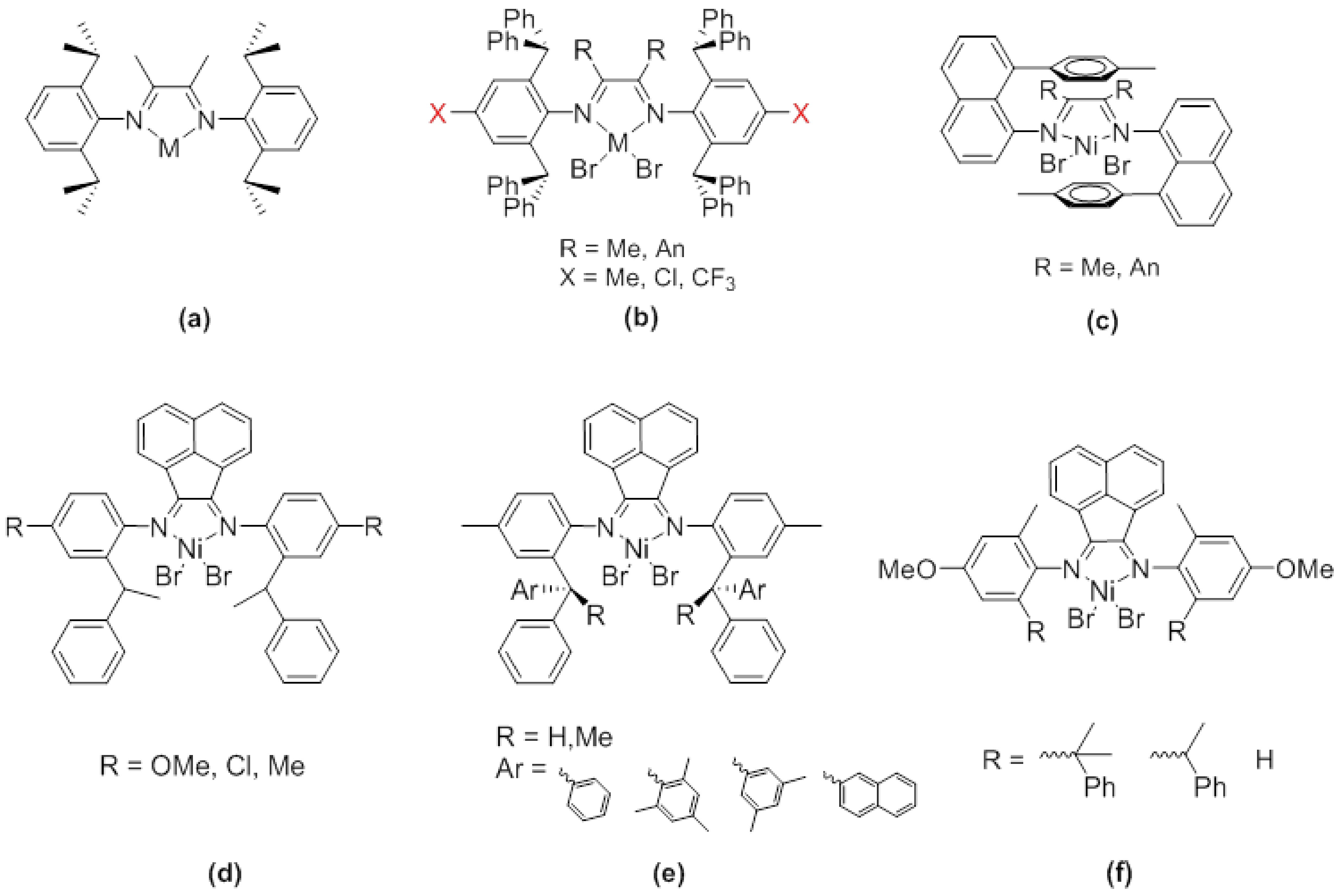
α-Diimide catalysts have attracted widespread attention due to their unique chain walking characteristics. A series of α-diimide nickel/palladium catalysts with different electronic effects and steric hindrances were designed and synthesized for olefin polymerization. In this work, we synthesized a series of asymmetric α-diimide nickel complexes with different steric hindrances and used them for ethylene polymerization. These nickel catalysts have high ethylene polymerization activity, up to 6.51×106 g·mol−1·h−1, and the prepared polyethylene has a moderate melting point and high molecular weight (up to 38.2 × 104 g·mol−1), with a branching density distribution between 7 and 94 branches per 1000 carbons. More importantly, the polyethylene prepared by these catalysts exhibits excellent tensile properties, with strain and stress reaching 800% and 30 MPa, respectively.
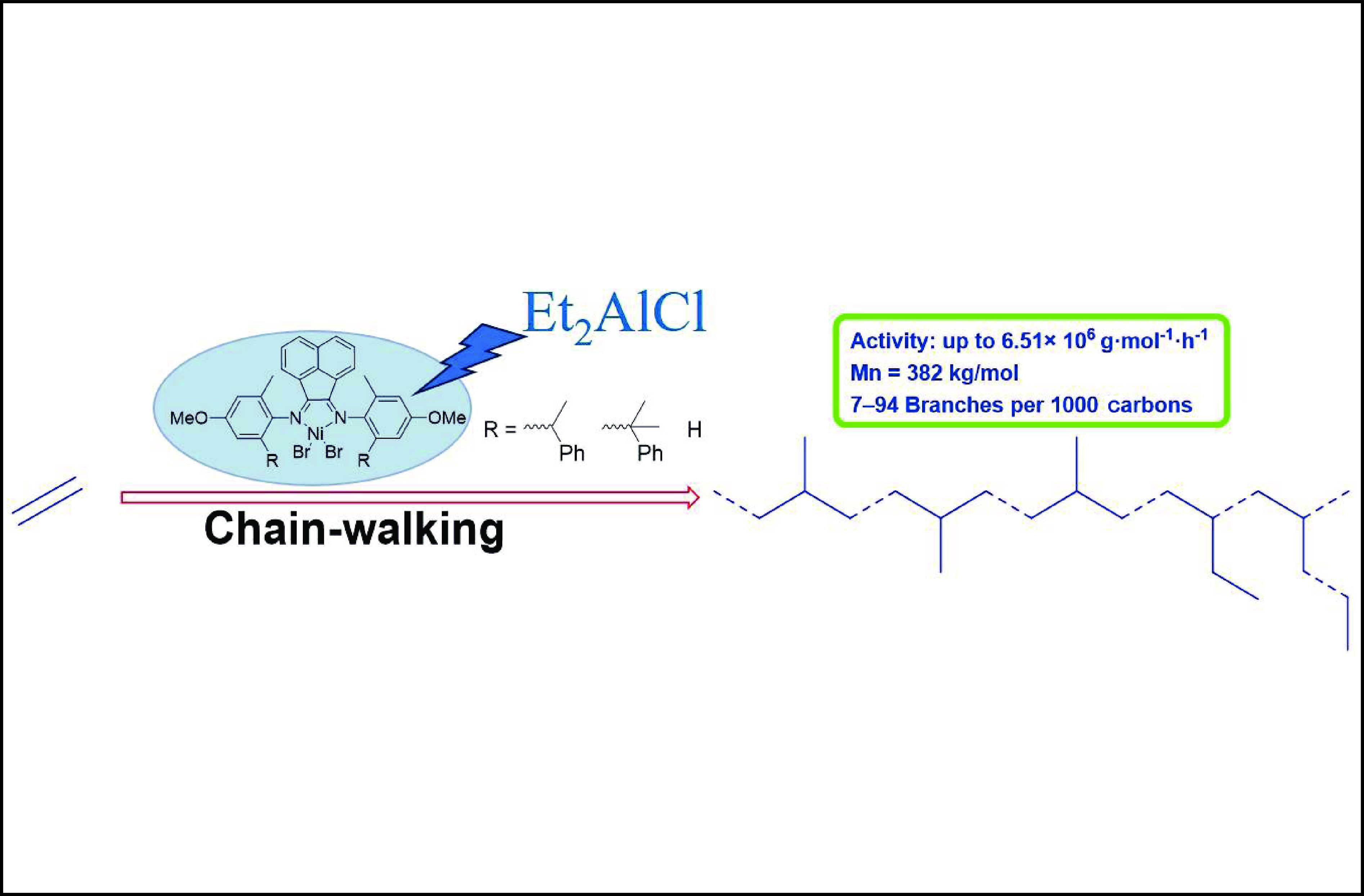
Synthesis of branched polyethylene by ethylene polymerization catalyzed by nickel catalysts with asymmetric steric hindrance.
Figure 2. Crystal structure of Ni1. The selected bond lengths (Å) and angles (°) were as follows: Br2–Ni1 = 2.3442(13), Br1–Ni1 = 2.3577(13), Ni1–N1 = 2.043(5), Ni1–N2 = 0.055(6), Br2–Ni1–Br1 = 110.57(5), N1–Ni1–N2 = 83.0(2), N1–Ni1–Br2 = 97.94(17), N1–Ni1–Br1 = 134.85(18), and N2–Ni1–Br1 = 99.18(16).
Figure 3. Crystal structure of Ni2. The selected bond lengths (Å) and angles (°) are as follows: Br1–Ni1 = 2.3451(9), Ni1–Br2 = 2.3256(9), Ni1–N2 = 2.020(4), Ni1–N1 = 2.020(4), Br2–Ni1–Br1 = 122.07(4), N2–Ni1–Br1 = 115.23(10), N2–Ni1–Br2 = 107.94(10), N1–Ni1–Br1 = 101.99(10), N1–Ni1–Br2 = 120.38(10), and N1–Ni1–N2 = 82.98(15).
Figure 4. The polymerization performance of catalysts. (a) Polymer weight comparisons of generated polyethylene at 0 °C, 30 °C, 60 °C and 90 °C. (b) Molecular weight comparisons of generated polyethylene at 0 °C, 30 °C, 60 °C and 90 °C. (c) Branching density comparisons of generated polyethylene at 0 °C, 30 °C, 60 °C and 90 °C. (d) Time-dependent studies (polymer yields vs polymerization time) at 90 °C.
| [1] |
Ittel S D, Johnson L K, Brookhart M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev., 2000, 100 (4): 1169–1204. DOI: 10.1021/cr9804644
|
| [2] |
Natta G, Pino P, Corradini P, et al. Crystalline high polymers of α-olefins. J. Am. Chem. Soc., 1955, 77 (6): 1708–1710. DOI: 10.1021/ja01611a109
|
| [3] |
Ziegler K, Holzkamp E, Breil H, et al. Polymerisation von Äthylen und anderen Olefinen. Angew. Chem., 1955, 67 (16): 426. DOI: 10.1002/ange.19550671610
|
| [4] |
Galli P, Vecellio G. Technology: driving force behind innovation and growth of polyolefins. Prog. Polym. Sci., 2001, 26 (8): 1287–1336. DOI: 10.1016/S0079-6700(01)00029-6
|
| [5] |
Mu H, Pan L, Song D, et al. Neutral nickel catalysts for olefin homo- and copolymerization: relationships between catalyst structures and catalytic properties. Chem. Rev., 2015, 115 (22): 12091–12137. DOI: 10.1021/cr500370f
|
| [6] |
Nakamura A, Anselment T M J, Claverie J, et al. Ortho-phosphinobenzenesulfonate: a superb ligand for palladium-catalyzed coordination-insertion copolymerization of polar vinyl monomers. Acc. Chem. Res., 2013, 46 (7): 1438–1449. DOI: 10.1021/ar300256h
|
| [7] |
Sun Y, Chi M J, Bashir M S, et al. Influence of intramolecular π–π and H-bonding interactions on pyrazolylimine nickel-catalyzed ethylene polymerization and co-polymerization. New J. Chem., 2021, 45 (30): 13280–13285. DOI: 10.1039/D1NJ02437J
|
| [8] |
Younkin T R, Connor E F, Henderson J I, et al. Neutral, single-component nickel (II) polyolefin catalysts that tolerate heteroatoms. Science, 2000, 287 (5452): 460–462. DOI: 10.1126/science.287.5452.460
|
| [9] |
Zou C, Dai S Y, Chen C L. Ethylene polymerization and copolymerization using nickel 2-iminopyridine- N-oxide catalysts: modulation of polymer molecular weights and molecular-weight distributions. Macromolecules, 2018, 51 (1): 49–56. DOI: 10.1021/acs.macromol.7b02156
|
| [10] |
Wang Q, Wang W B, Qu W C, et al. Ethylene homo and copolymerization by phosphorus-benzoquinone based homogeneous and heterogeneous nickel catalysts. J. Polym. Sci., 2023, 61 (2): 115–122. DOI: 10.1002/pol.20220381
|
| [11] |
Klabunde U, Itten S D. Nickel catalysis for ethylene homo- and co-polymerization. J. Mol. Catal., 1987, 41 (1/2): 123–134. DOI: 10.1016/0304-5102(87)80023-8
|
| [12] |
Wang W B, Chen M, Pang W M, et al. Palladium-catalyzed synthesis of norbornene-based polar-functionalized polyolefin elastomers. Macromolecules, 2021, 54 (7): 3197–3203. DOI: 10.1021/acs.macromol.1c00201
|
| [13] |
Johnson L K, Killian C M, Brookhart M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc., 1995, 117 (23): 6414–6415. DOI: 10.1021/ja00128a054
|
| [14] |
Hu X Q, Kang X H, Jian Z B. Suppression of chain transfer at high temperature in catalytic olefin polymerization. Angew. Chem. Int. Ed., 2022, 61 (33): e202207363 DOI: 10.1002/anie.202207363
|
| [15] |
Zheng H D, Li Y W, Du W B, et al. Unprecedented square-planar α-diimine dibromonickel complexes and their ethylene polymerizations modulated by Ni–phenyl interactions. Macromolecules, 2022, 55 (9): 3533–3540 DOI: 10.1021/acs.macromol.2c00360
|
| [16] |
Lu W Q, Ding B H, Zou W P, et al. Direct synthesis of polyethylene thermoplastic elastomers with high molecular weight and excellent elastic recovery via a hybrid steric bulky strategy. Eur. Poly. J., 2023, 201 (11): 112577 DOI: 10.1016/j.eurpolymj.2023.112577
|
| [17] |
Ji H Y, Zhang Y X, Chi Y, et al. Concerted flexible and steric strategy in α-diimine nickel and palladium mediated insertion (Co-)Polymerization. Polymer, 2024, 290: 126591. DOI: 10.1016/j.polymer.2023.126591
|
| [18] |
Rhinehart J L, Brown L A, Long B K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc., 2013, 135 (44): 16316–16319. DOI: 10.1021/ja408905t
|
| [19] |
Dai S Y, Sui X L, Chen C L. Highly robust palladium(II) α–diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed., 2015, 54 (34): 9948–9953. DOI: 10.1002/anie.201503708
|
| [20] |
Padilla-Vélez O, O’Connor K S, LaPointe A M, et al. Switchable living nickel(II) α-diimine catalyst for ethylene polymerisation. Chem. Commun., 2019, 55 (53): 7607–7610. DOI: 10.1039/C9CC03154E
|
| [21] |
Wang F Z, Yuan J C, Li Q S, et al. New nickel(II) diimine complexes bearing phenyl and sec-phenethyl groups: synthesis, characterization and ethylene polymerization behaviour. Appl. Organomet. Chem., 2014, 28 (7): 477–483. DOI: 10.1002/aoc.3151
|
| [22] |
Rose J M, Deplace F, Lynd N A, et al. C2-symmetric Ni(II) α-diimines featuring cumyl-derived ligands: synthesis of improved elastomeric regioblock polypropylenes. Macromolecules, 2008, 41 (24): 9548–9555. DOI: 10.1021/ma8019943
|
| [23] |
Cherian A E, Rose J M, Lobkovsky E B, et al. A C2-symmetric, living α-diimine Ni(II) catalyst: regioblock copolymers from propylene. J. Am. Chem. Soc., 2005, 127 (40): 13770–13771. DOI: 10.1021/ja0540021
|
| [24] |
Vaccarello D N, O’Connor K S, Iacono P, et al. Synthesis of semicrystalline polyolefin materials: precision methyl branching via stereoretentive chain walking. J. Am. Chem. Soc., 2018, 140 (20): 6208–6211. DOI: 10.1021/jacs.8b02963
|
| Entry | Catalyst |
T(°C) | Yieldb (g) | Activityb (106) | Mnc(104) | PDIc |
Branchd |
Tme(°C) |
| 1 | Ni1 | 0 | 2.65 | 3.18 | 38.2 | 1.8 | 7 | 128.2 |
| 2 | Ni1 | 30 | 5.43 | 6.51 | 33.6 | 2.1 | 21 | 118.0 |
| 3 | Ni1 | 60 | 2.52 | 3.02 | 21.9 | 2.0 | 40 | 114.4 |
| 4 | Ni1 | 90 | 2.01 | 2.41 | 16.6 | 2.1 | 41 | 114.0 |
| 5 | Ni2 | 0 | 2.35 | 2.82 | 37.6 | 1.8 | 15 | 120.2 |
| 6 | Ni2 | 30 | 4.11 | 4.93 | 29.7 | 1.9 | 26 | 117.0 |
| 7 | Ni2 | 60 | 2.76 | 3.31 | 17.3 | 2.1 | 46 | 113.6 |
| 8 | Ni2 | 90 | 0.90 | 1.08 | 14.8 | 2.1 | 61 | 80.9 |
| 9 | Ni3 | 0 | 1.10 | 1.32 | 16.2 | 2.3 | 36 | 115.1 |
| 10 | Ni3 | 30 | 1.94 | 2.33 | 12.8 | 2.6 | 51 | 106.1 |
| 11 | Ni3 | 60 | 0.80 | 0.96 | 11.9 | 3.1 | 72 | 69.1 |
| 12 | Ni3 | 90 | 0.02 | 0.02 | 11.5 | 3.2 | 94 | – |
| a 1 μmol of catalyst in CH2Cl2 (2 mL), [Al]/[Ni] = 500. Vn-heptane = 20 mL, tpolymerization = 10 min, Pethylene = 8 atm. b Activity is in units of 106 g·mol−1·h−1. c Determined by Gel Permeation Chromatography (GPC) in 1,2,4-trichlorobenzene at 150 °C. d Branches per 1000 carbons, determined by 1H NMR. e Determined by differential scanning calorimetry. | ||||||||