ISSN 0253-2778
CN 34-1054/N
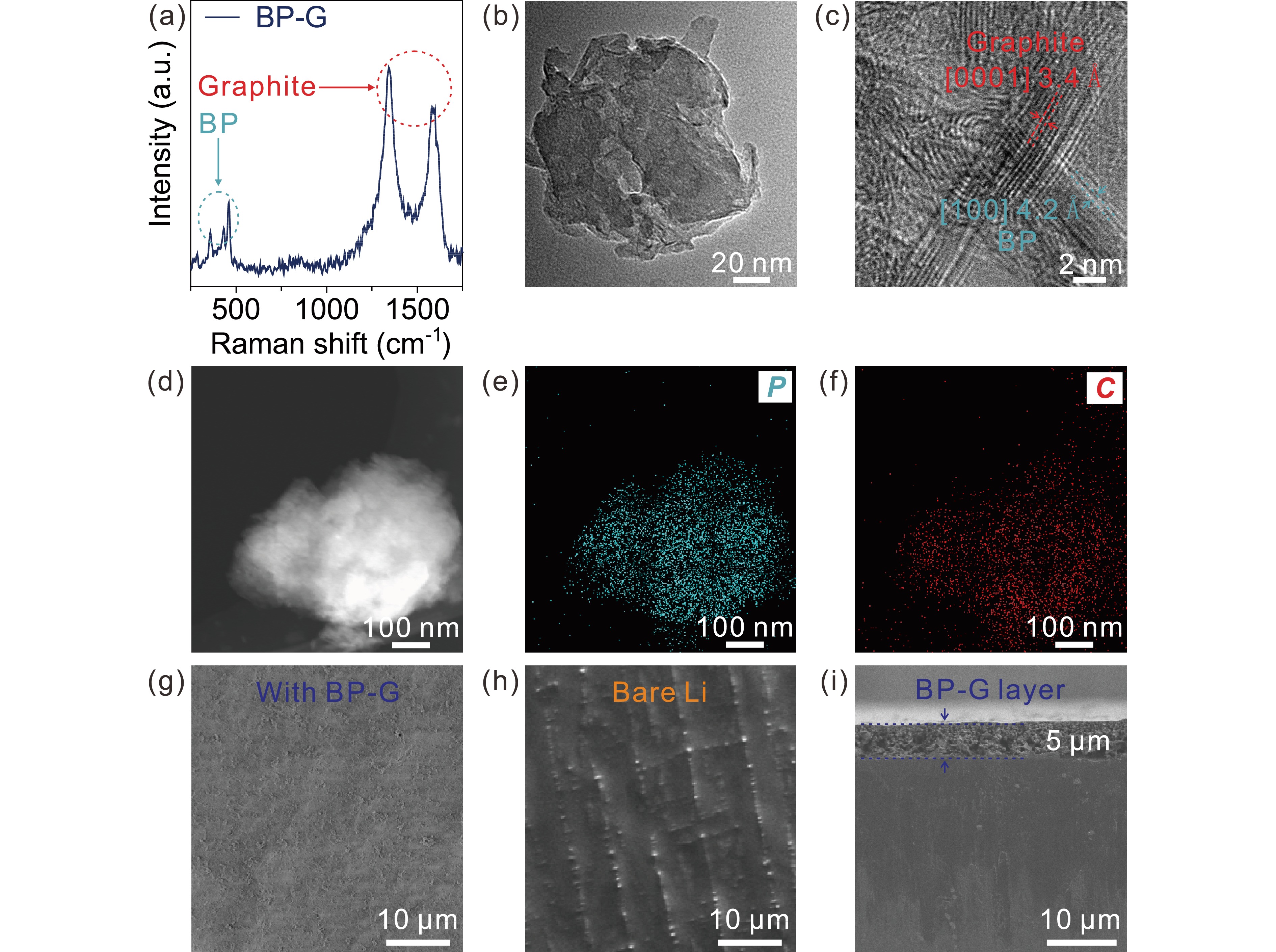
Lithium (Li) metal anodes have been regarded as the most promising candidates for high energy density secondary lithium batteries due to their high specific capacity and low redox potential. However, the issues of Li dendrites caused by nonuniform lithium deposition during battery cycling severely hinder the practical applications of Li metal anodes. Herein, a hybrid of black phosphorus-graphite (BP-G) is introduced to serve as an artificial protective layer for the Li metal anode. The two-dimensional few-layer BP, which is lithophilic, combined with the high electronic conductive graphite can act as a regulator to adjust the migration of Li ions, delivering a uniform and stable lithium deposition. As the growth of lithium dendrites is inhibited, the utilization of Li metal achieves > 98.5% for over 500 cycles in Li||Cu half cells, and the life span is maintained over 2000 h in Li||Li symmetric cells with a low voltage hysteresis of 50 mV. Moreover, the LiFePO4||Li full cell with a BP-G Li-ion regulator presents significantly better specific capacity and cycling stability than that with the bare Li metal anode. Therefore, the introduction of the BP-G Li-ion regulator is demonstrated to be an effective approach to enable stable lithium deposition for rechargeable Li metal batteries.
A black phosphorus-graphite hybrid acts as the Li-ion regulator, enabling stable lithium deposition.
Figure 2. Li-ion transportation behaviors. (a) Tafel curves of the Li||Li symmetric cells with or without the BP-G layer to evaluate the exchange current densities. (b) Short circuit tests of Li||Cu half cells with bare Li or (BP-G)/Li metal anodes at 1.0 mA∙cm−2. Nyquist plots of Li||Li symmetric cells before and after polarization with the (c) bare Li and (d) (BP-G)/Li metal anodes; the corresponding direct current polarization curves of the (e) bare Li and (f) (BP-G)/Li metal anodes.
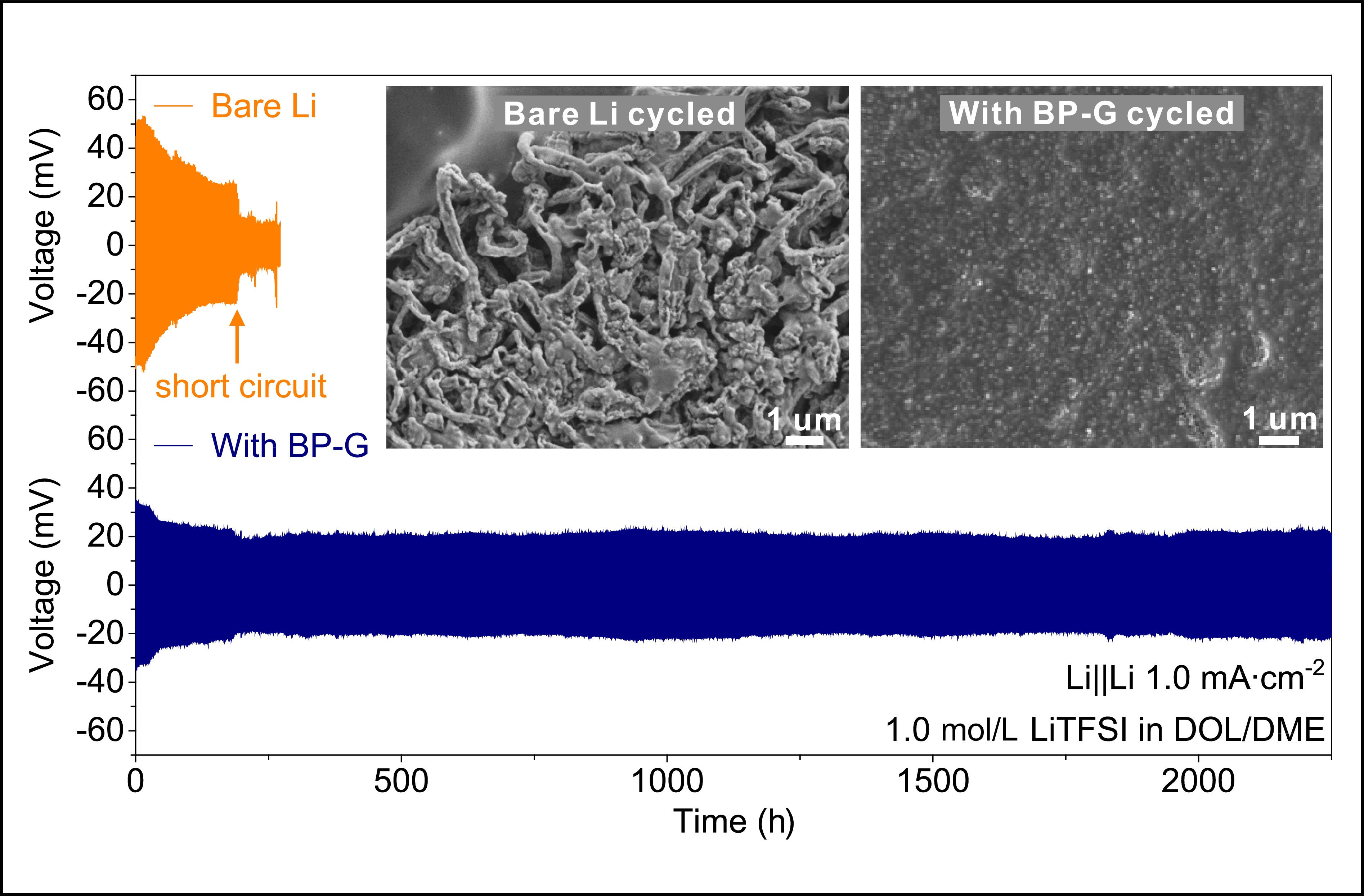
Figure 3. Electrochemical performances of Li||Cu half cells and Li||Li symmetric cells. (a) The variation of CE values in Li||Cu cells with the bare Li or (BP-G)/Li metal anodes at the current density of 1.0 mA∙cm−2 and areal capacity of 1.0 mA∙h∙cm−2. The corresponding charge/discharge curves at the (b) 50th and (c) 100th cycles. (d) Voltage profiles of Li||Li symmetric cells with the bare Li (orange line) and (BP-G)/Li (blue line) metal anodes at a current density of 1.0 mA∙cm−2 and an areal capacity of 1.0 mA∙h∙cm−2. The insets show the corresponding enlarged voltage profiles at different cycling stages. Impedance spectra of Li||Li symmetric cells with the bare Li or (BP-G)/Li metal anodes at (e) the initial state and the end of the (f) 100th and (g) 1000th cycles.
Figure 4. Electrochemical performances of LFP||Li full cells. (a) Variation in specific capacity retention with cycle numbers at 1.0 C. (b) Charge/discharge voltage profiles of the 10th and 300th cycles. Morphologies of lithium metal anodes after 300 cycles with the (c) bare Li or (d) (BP-G)/Li metal anodes.
| [1] |
Sheng L, Wang Q, Liu X, et al. Suppressing electrolyte-lithium metal reactivity via Li(+)-desolvation in uniform nano-porous separator. Nat. Commun., 2022, 13 (1): 172. DOI: 10.1038/s41467-021-27841-0
|
| [2] |
Winter M, Barnett B, Xu K. Before Li ion batteries. Chem. Rev., 2018, 118 (23): 11433–11456. DOI: 10.1021/acs.chemrev.8b00422
|
| [3] |
Wang M, Emre A E, Kim J Y, et al. Multifactorial engineering of biomimetic membranes for batteries with multiple high-performance parameters. Nat. Commun., 2022, 13 (1): 278. DOI: 10.1038/s41467-021-27861-w
|
| [4] |
Yao Y X, Zhang X Q, Li B Q, et al. A compact inorganic layer for robust anode protection in lithium‐sulfur batteries. InfoMat, 2020, 2 (2): 379–388. DOI: 10.1002/inf2.12046
|
| [5] |
Li J, Kong Z, Liu X, et al. Strategies to anode protection in lithium metal battery: A review. InfoMat, 2021, 3 (12): 1333–1363. DOI: 10.1002/inf2.12189
|
| [6] |
Han Z, Zhang C, Lin Q, et al. A protective layer for lithium metal anode: Why and how. Small Methods, 2021, 5 (4): 2001035. DOI: 10.1002/smtd.202001035
|
| [7] |
Ye Y, Zhao Y, Zhao T, et al. An Antipulverization and high-continuity lithium metal anode for high-energy lithium batteries. Adv. Mater., 2021, 33 (49): 2105029. DOI: 10.1002/adma.202105029
|
| [8] |
Gao Y, Rojas T, Wang K, et al. Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat. Energy, 2020, 5 (7): 534–542. DOI: 10.1038/s41560-020-0640-7
|
| [9] |
Wang Q, Yang C, Yang J, et al. Dendrite-free lithium deposition via a superfilling mechanism for high-performance Li-metal batteries. Adv. Mater., 2019, 31 (41): 1903248. DOI: 10.1002/adma.201903248
|
| [10] |
Zheng J, Yan P, Mei D, et al. Highly stable operation of lithium metal batteries enabled by the formation of a transient high-concentration electrolyte layer. Adv. Energy Mater., 2016, 6 (8): 1502151. DOI: 10.1002/aenm.201502151
|
| [11] |
Huang M, Yao Z, Yang Q, et al. Consecutive nucleation and confinement modulation towards Li plating in seeded capsules for durable Li-metal batteries. Angew. Chem. Int. Ed., 2021, 60 (25): 14040–14050. DOI: 10.1002/anie.202102552
|
| [12] |
Xu Y, Zhou Y, Li T, et al. Multifunctional covalent organic frameworks for high capacity and dendrite-free lithium metal batteries. Energy Stor. Mater., 2020, 25: 334–341. DOI: 10.1016/j.ensm.2019.10.005
|
| [13] |
Chang Z, Qiao Y, Yang H, et al. Sustainable lithium-metal battery achieved by a safe electrolyte based on recyclable and low-cost molecular sieve. Angew. Chem. Int. Ed., 2021, 60 (28): 15572–15581. DOI: 10.1002/anie.202104124
|
| [14] |
Lange S, Schmidt P, Nilges T. Au3SnP7@ black phosphorus: An easy access to black phosphorus. Inorg. Chem., 2007, 46 (10): 4028–4035. DOI: 10.1021/ic062192q
|
| [15] |
Sun J, Sun Y, Pasta M, et al. Entrapment of polysulfides by a black-phosphorus-modified separator for lithium-sulfur batteries. Adv. Mater., 2016, 28 (44): 9797–9803. DOI: 10.1002/adma.201602172
|
| [16] |
Jin H, Xin S, Chuang C, et al. Black phosphorus composites with engineered interfaces for high-rate high-capacity lithium storage. Science, 2020, 370: 192–197. DOI: 10.1126/science.aav5842
|
| [17] |
Zhao C Z, Chen P Y, Zhang R, et al. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv., 2018, 4 (11): eaat3446. DOI: 10.1126/sciadv.aat3446
|
| [18] |
Chang Z, Qiao Y, Deng H, et al. A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule, 2020, 4 (8): 1776–1789. DOI: 10.1016/j.joule.2020.06.011
|
| [19] |
He X, Jin S, Miao L, et al. A 3D hydroxylated MXene/carbon nanotubes composite as a scaffold for dendrite-free sodium-metal electrodes. Angew. Chem. Int. Ed., 2020, 59 (38): 16705–16711. DOI: 10.1002/anie.202006783
|
| [20] |
Xie H, Hao Q, Jin H, et al. Redistribution of Li-ions using covalent organic frameworks towards dendrite-free lithium anodes: A mechanism based on a Galton Board. Sci. China Chem., 2020, 63 (9): 1306–1314. DOI: 10.1007/s11426-020-9796-9
|
| [21] |
He Y, Chang Z, Wu S, et al. Simultaneously inhibiting lithium dendrites growth and polysulfides shuttle by a flexible MOF-based membrane in Li-S batteries. Adv. Energy Mater., 2018, 8 (34): 1802130. DOI: 10.1002/aenm.201802130
|
| [22] |
Tang X, Zhou D, Li P, et al. MXene-based dendrite-free potassium metal batteries. Adv. Mater., 2020, 32 (4): 1906739. DOI: 10.1002/adma.201906739
|