
ISSN 0253-2778
CN 34-1054/N
A three-dimensional elastic carbon nanotube aerogel is fabricated via a simple solution-based strategy using Te nanowires as templates, which can be recycled. The pipe diameter and wall thickness of the carbon nanotube are strongly dependent on the diameter of the Te nanowires and carbon source. The obtained free-standing carbon nanotube aerogel with a large specific surface area (up to 1865 m2∙g-1) is promising as an electrode material for supercapacitors. After combination with MnO2, the capacitor exhibits a specific capacitance of 360.4 F∙g-1 at a current of 1 A∙g-1 and retention of 97% after 2000 cycles. The high power capabilities and good stability make it a promising candidate as an electrode for supercapacitors.
Carbon nanotube gels with large specific surface areas are promising for use as electrode materials for supercapacitors.
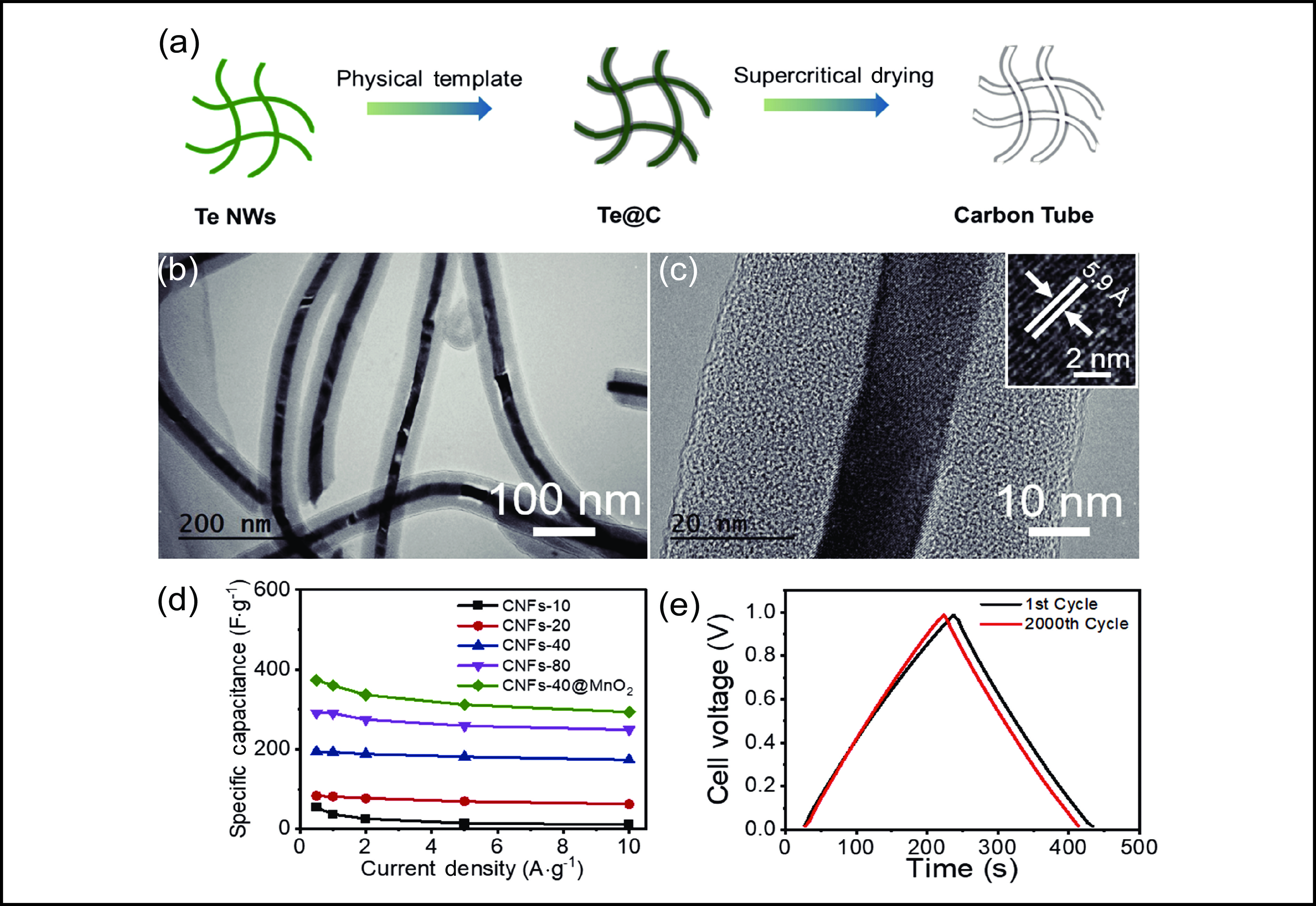
Figure 1. (a) Schematic of the typical synthesis of the carbon tube. (b,c) TEM images of the Te@C fiber (Te NWs coated with a uniform carbon shell) at different magnifications. The inset of (c) shows a HRTEM image of the inner Te NW. (d–f) Maps of the Te@C nanocable. (g) Corresponding selected-area electron diffraction pattern of a Te@C nanowire cable.
Figure
2.
Morphology control of the tubular carbon aerogel.
Figure 3. Electrochemical performances measured in a two-electrode system. Cyclic voltammograms of the carbon nanotube-10, carbon nanotube-20, carbon nanotube-40, and carbon nanotube-80 at scan rates of (a) 50 mV∙s−1 and (b) 100 mV∙s−1. (c) Galvanostatic charge–discharge curves at 1 A∙g−1. (d) Volumetric capacitances at different current densities.
Figure 5. Electrochemical performances measured in a two-electrode system. Cyclic voltammograms of the MnO2@carbon nanotube-40 at scan rates of 50 and 100 mV∙s −1. (b) Galvanostatic charge–discharge curves of the MnO2@carbon nanotube-40 at different current densities. (c) Volumetric capacitances of the MnO2@ carbon nanotube-40 and carbon nanotube-x (x = 10, 20, 40, 80) at different current densities. (d) Triangular shapes of the first and 2000th cycles.
| [1] |
Zhai Y P, Dou Y Q, Zhao D Y, et al. Carbon materials for chemical capacitive energy storage. Adv. Mater., 2011, 23 (42): 4828–4850. DOI: 10.1002/adma.201100984
|
| [2] |
Zhu Y, Murali S, Stoller M D, et al. Carbon-based supercapacitors produced by activation of graphene. Science, 2011, 332 (6037): 1537–1541. DOI: 10.1126/science.1200770
|
| [3] |
Zhou D, Cui Y, Han B H. Graphene-based hybrid materials and their applications in energy storage and conversion. Chin. Sci. Bull., 2012, 57 (23): 2983–2994. DOI: 10.1007/s11434-012-5314-9
|
| [4] |
Zheng G Y, Lee S W, Liang Z, et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol., 2014, 9 (8): 618–623. DOI: 10.1038/nnano.2014.152
|
| [5] |
Lou X W, Archer L A, Yang Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater., 2008, 20 (21): 3987–4019. DOI: 10.1002/adma.200800854
|
| [6] |
Liu C, Li F, Ma L P, et al. Advanced materials for energy storage. Adv. Mater., 2010, 22 (8): E28–E62. DOI: 10.1002/adma.200903328
|
| [7] |
Li H, Wang Z X, Chen L Q, et al. Research on advanced materials for Li-ion batteries. Adv. Mater., 2009, 21 (45): 4593–4607. DOI: 10.1002/adma.200901710
|
| [8] |
Arico A S, Bruce P, Scrosati B, et al. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater., 2005, 4 (5): 366–377. DOI: 10.1038/nmat1368
|
| [9] |
Yu X W, Manthiram A. Ambient-temperature sodium-sulfur batteries with a sodiated Nafion membrane and a carbon nanofiber-activated carbon composite electrode. Adv. Energy. Mater., 2015, 5 (12): 1500350. DOI: 10.1002/aenm.201500350
|
| [10] |
McDonough J R, Choi J W, Yang Y, et al. Carbon nanofiber supercapacitors with large areal capacitances. Appl. Phys. Lett., 2009, 95 (24): 243109. DOI: 10.1063/1.3273864
|
| [11] |
Kim T, Jung G, Yoo S, et al. Activated graphene-based carbons as supercapacitor electrodes with macro- and mesopores. ACS Nano, 2013, 7 (8): 6899–6905. DOI: 10.1021/nn402077v
|
| [12] |
Kim H, Cho M Y, Kim M H, et al. A novel high-energy hybrid supercapacitor with an anatase TiO2-reduced graphene oxide anode and an activated carbon cathode. Adv. Energy. Mater., 2013, 3 (11): 1500–1506. DOI: 10.1002/aenm.201300467
|
| [13] |
Wang G M, Wang H Y, Lu X H, et al. Solid-state supercapacitor based on activated carbon cloths exhibits excellent rate capability. Adv. Mater., 2014, 26 (17): 2676–2682. DOI: 10.1002/adma.201304756
|
| [14] |
Kaempgen M, Chan C K, Ma J, et al. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett., 2009, 9 (5): 1872–1876. DOI: 10.1021/nl8038579
|
| [15] |
Futaba D N, Hata K, Yamada T, et al. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater., 2006, 5 (12): 987–994. DOI: 10.1038/nmat1782
|
| [16] |
Frackowiak E, Beguin F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon, 2002, 40 (10): 1775–1787. DOI: 10.1016/S0008-6223(02)00045-3
|
| [17] |
De Volder M F L, Tawfick S H, Baughman R H, et al. Carbon nanotubes: Present and future commercial applications. Science, 2013, 339 (6119): 535–539. DOI: 10.1126/science.1222453
|
| [18] |
Che G L, Lakshmi B B, Fisher E R, et al. Carbon nanotubule membranes for electrochemical energy storage and production. Nature, 1998, 393 (6683): 346–349. DOI: 10.1038/30694
|
| [19] |
Wang D W, Li F, Liu M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed., 2007, 47 (2): 373–376. DOI: 10.1002/anie.200702721
|
| [20] |
Cheng F Y, Tao Z L, Liang J, et al. Template-directed materials for rechargeable lithium-ion batteries. Chem. Mater., 2008, 20 (3): 667–681. DOI: 10.1021/cm702091q
|
| [21] |
Yoo E, Kim J, Hosono E, et al. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett., 2008, 8 (8): 2277–2282. DOI: 10.1021/nl800957b
|
| [22] |
Wang D, Choi D, Li J, et al. Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano, 2009, 3 (4): 907–914. DOI: 10.1021/nn900150y
|
| [23] |
Chen S, Zhu J W, Wu X D, et al. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano, 2010, 4 (5): 2822–2830. DOI: 10.1021/nn901311t
|
| [24] |
Reddy A L M, Srivastava A, Gowda S R, et al. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano, 2010, 4 (11): 6337–6342. DOI: 10.1021/nn101926g
|
| [25] |
Wu Z S, Ren W C, Xu L, et al. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano, 2011, 5 (7): 5463–5471. DOI: 10.1021/nn2006249
|
| [26] |
Zhang L L, Zhao X S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev., 2009, 38 (9): 2520–2531. DOI: 10.1039/B813846J
|
| [27] |
Qian H S, Yu S H, Gong J Y, et al. High-quality luminescent tellurium nanowires of several nanometers in diameter and high aspect ratio synthesized by a poly (vinyl pyrrolidone)-assisted hydrothermal process. Langmuir, 2006, 22 (8): 3830–3835. DOI: 10.1021/la053021l
|
| [28] |
Liang H W, Wang L, Chen P Y, et al. Carbonaceous nanofiber membranes for selective filtration and separation of nanoparticles. Adv. Mater., 2010, 22 (42): 4691–4695. DOI: 10.1002/adma.201001863
|
| [29] |
Fan Z J, Yan J, Wei T, et al. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater., 2011, 21 (12): 2366–2375. DOI: 10.1002/adfm.201100058
|
| [30] |
Wei W, Cui X, Chen W, et al. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev., 2011, 40 (3): 1697–1721. DOI: 10.1039/C0CS00127A
|
| [31] |
Yu G, Hu L, Vosgueritchian M, et al. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett., 2011, 11 (7): 2905–2911. DOI: 10.1021/nl2013828
|
| [32] |
Wu Z S, Ren W C, Wang D W, et al. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano, 2010, 4 (10): 5835–5842. DOI: 10.1021/nn101754k
|
| [33] |
Guo C X, Wang M, Chen T, et al. A hierarchically nanostructured composite of MnO2/conjugated polymer/graphene for high-performance lithium ion batteries. Adv. Energy. Mater., 2011, 1 (5): 736–741. DOI: 10.1002/aenm.201100223
|
| [34] |
Lyu X M, Su F H, Miao M H. Two-ply yarn supercapacitor based on carbon nanotube/stainless steel core-sheath yarn electrodes and ionic liquid electrolyte. J. Power Sources, 2016, 307: 489–495. DOI: 10.1016/j.jpowsour.2015.12.114
|
| [35] |
He S S, Hu Y J, Wan J X, et al. Biocompatible carbon nanotube fibers for implantable supercapacitors. Carbon, 2017, 122: 162–167. DOI: 10.1016/j.carbon.2017.06.053
|
| [36] |
Wan L, Shamsaei E, Easton C D, et al. ZIF-8 derived nitrogen-doped porous carbon/carbon nanotube composite for high-performance supercapacitor. Carbon, 2017, 121: 330–336. DOI: 10.1016/j.carbon.2017.06.017
|
| [37] |
Rong K, Wei J L, Wang Y C, et al. Deep eutectic solvent assisted zero-waste electrospinning of lignin fiber aerogels. Green Chem., 2021, 23 (16): 6065–6075. DOI: 10.1039/D1GC01872H
|
| [38] |
Chakraborty S, Simon R, Vadakkekara A, et al. Microwave assisted synthesis of poly(ortho-phenylenediamine-co-aniline) and functionalised carbon nanotube nanocomposites for fabric-based supercapacitors. Electrochim. Acta, 2022, 403: 139678. DOI: 10.1016/j.electacta.2021.139678
|
| Category | Value |
| Ambient temperature (℃) | 18.0 |
| Environmental pressure (MPa) | 0.1 |
| Inlet pressure (MPa) | 0.1 |
| Working fluid flow (L·h−1) | 53.0 |
| Inlet temperature (℃) | 15.0 |
| Nozzle diameter (mm) | 0.3 |
| Types of additives | SDS, N-octanol, Tween20 |
| Additive concentration (ppm) | 100 / 200 / 300 / 400 |
| Parameters | Measurement error |
| Temperature (℃) | ± 0.5 |
| Pressure (MPa) | ± 0.002 |
| Voltage (V) | ± 0.5 |
| Flow (L·h−1) | ± 0.1 |
| Current (mA) | ± 1 |
| Length (mm) | ± 0.1 |
| Parameters | Uncertainty |
| q″ (W·cm−2) | ± 1.4% |
| h (W·cm−2·K−1) | ± 1.5% |
| Nu | ± 2.5% |
| Re | ± 0.7% |
| Pr | ± 2.5% |
| We | ± 0.2% |
| ε | ± 0.8% |
| Additives | coefficient | |||||
| A | B | C | D | E | F | |
| SDS | −1.815 | 1.167 | 0.089 | 0.150 | −4.891 | −0.253 |
| N-octanol | 2259 | 2.057 | 0.061 | 0.788 | −1.000 | 1.182 |
| Tween20 | 0.295 | 1.198 | −0.382 | 2.106 | 0.387 | 0.218 |