Figures of the Article
-
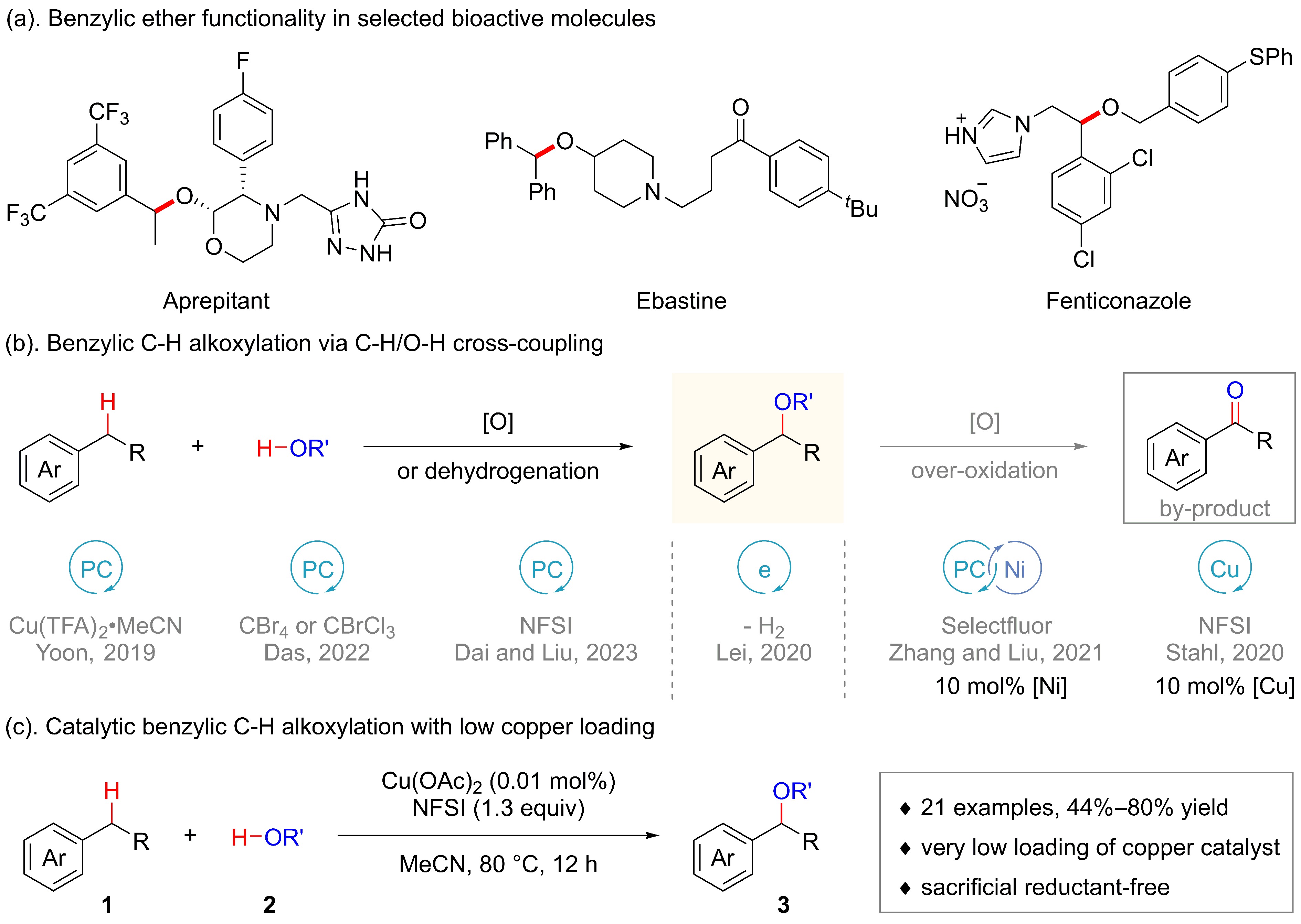
![]() Strategies toward benzylic C−H alkoxylation.
Strategies toward benzylic C−H alkoxylation.
-
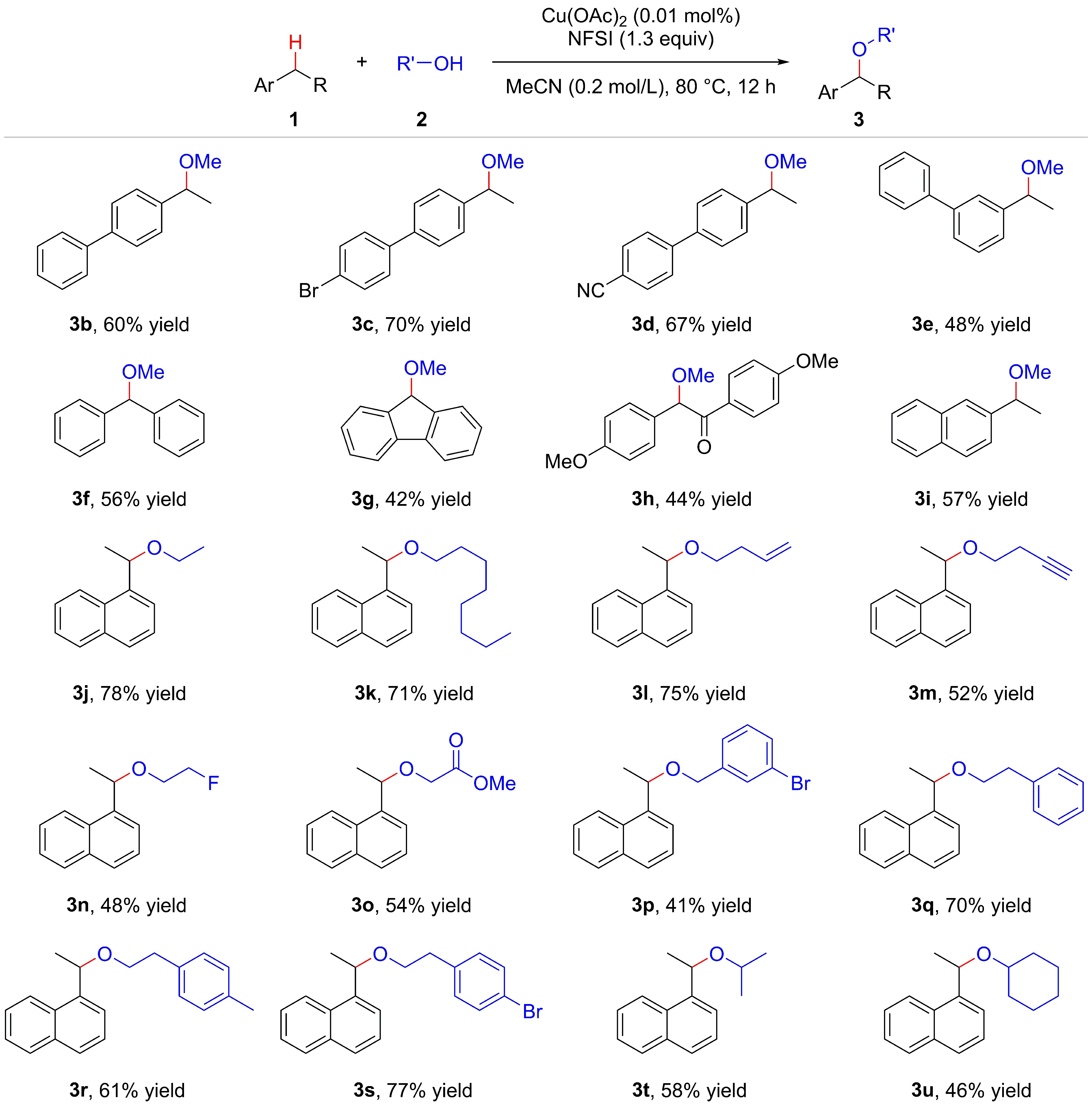
![]() Scope of substrates. Reaction conditions: alkylarene 1 (0.10 mmol), alcohol 2 (0.50 mmol), NFSI (0.13 mmol), Cu(OAc)2 (0.01 mol%), MeCN (0.5 mL), 80 °C, 12 h, under argon atmosphere. Isolated yield.
Scope of substrates. Reaction conditions: alkylarene 1 (0.10 mmol), alcohol 2 (0.50 mmol), NFSI (0.13 mmol), Cu(OAc)2 (0.01 mol%), MeCN (0.5 mL), 80 °C, 12 h, under argon atmosphere. Isolated yield.
-
![]() Kinetic studies.
Kinetic studies.
-
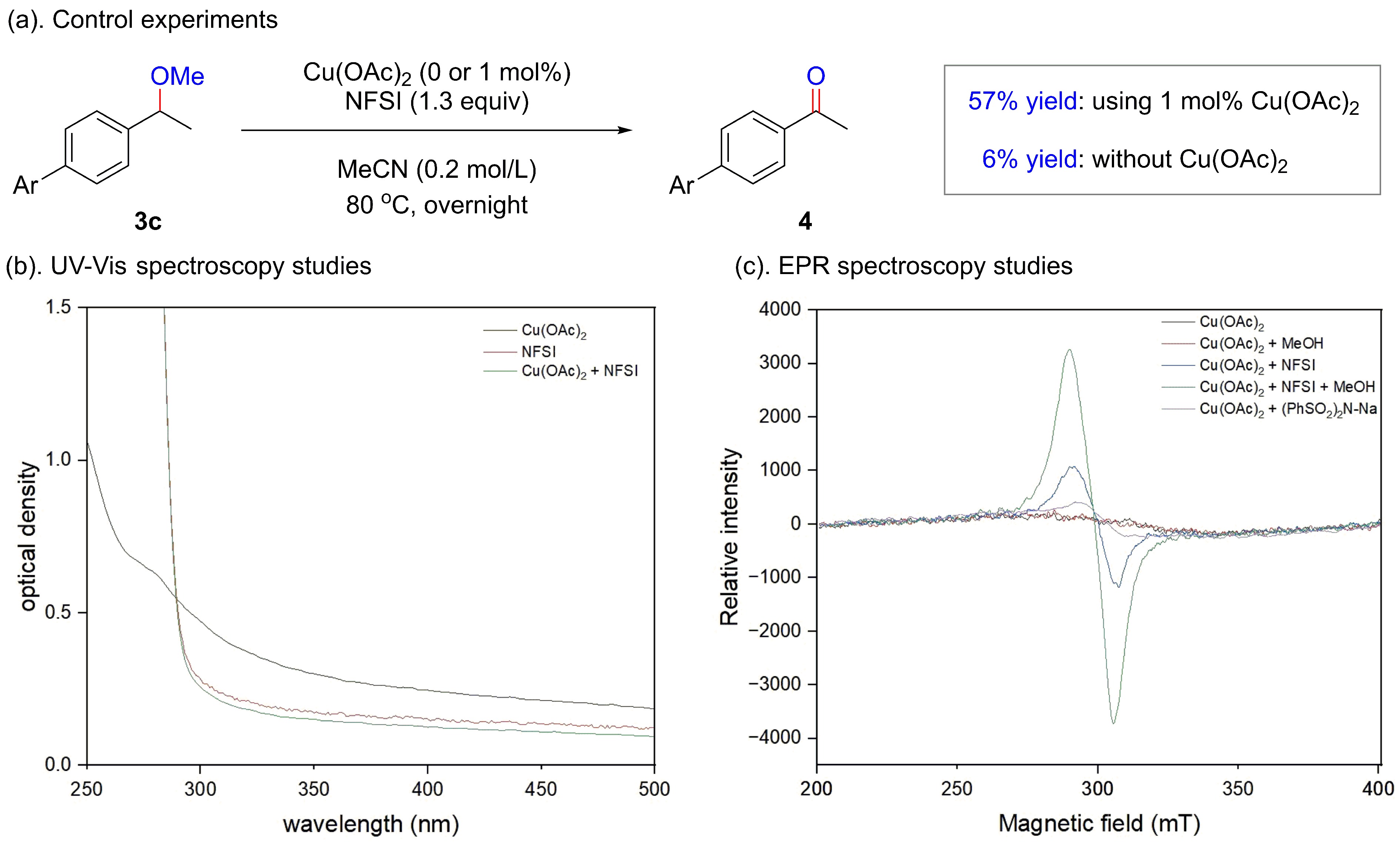
![]() Mechanistic studies.
Mechanistic studies.
-
![]() Proposed catalytic cycle.
Proposed catalytic cycle.
Related articles
-
2024, 54(7): 0704. DOI: 10.52396/JUSTC-2023-0155
-
2022, 52(8): 1-1-1-6. DOI: 10.52396/JUSTC-2022-0037
-
2022, 52(7): 2-1-2-11. DOI: 10.52396/JUSTC-2022-0004
-
2020, 50(6): 811-818. DOI: 10.3969/j.issn.0253-2778.2020.06.013
-
2019, 49(1): 79-86. DOI: 10.3969/j.issn.0253-2778.2019.01.011
-
2018, 48(8): 649-654. DOI: 10.3969/j.issn.0253-2778.2018.08.008
-
2018, 48(5): 374-377. DOI: 10.3969/j.issn.0253-2778.2018.05.005
-
Entangled state representation for mesoscopic persistent current ring and its phase-angular momentum2016, 46(8): 652-656. DOI: 10.3969/j.issn.0253-2778.2016.08.005
-
2014, 44(10): 839-843. DOI: 10.3969/j.issn.0253-2778.2014.10.007
-
2013, 43(5): 379-386. DOI: 10.3969/j.issn.0253-2778.2013.05.005



 Download:
Download: